General Directions for IRB Submission through Axiom Mentor
Please note- Lead investigators will not be able to access the portal to submit a protocol application until they have successfully completed the protection of human subjects training. As of July 1, 2019, Belmont University requires that all PIs, co-PIs, and faculty advisors of student research complete the CITI training. The information for accessing this training and the Belmont IRB training policy can be found out http://www.belmont.edu/irb/policies/citi-training.html.
CITI training certifications will not automatically upload into the Mentor system. Each person needs to upload their certificates under the PI documentation item in the left hand menu.
- Log into the Axiom Mentor System at https://www.axiommentor.com/login/shibLogin.cfm?i=belmont. The institution ID is belmont and you will use your MyBelmont credentials to access the system. Note: The Axiom Mentor System is the name of the online IRB protocol application management system.
- Also see the “Info Page” tab to the left of the screen. This “Info Page” will give you additional insight into the process of submitting an application, if needed.
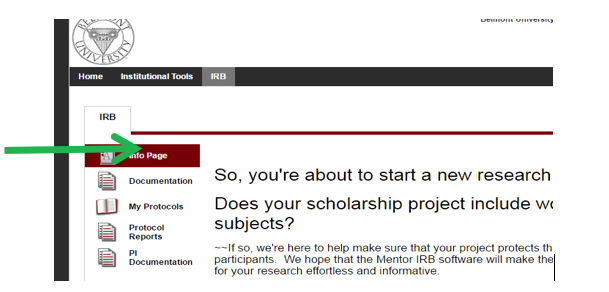
- Once you have completed the CITI training and outlined your research project and are ready to begin your IRB submission, click on the My Protocols tab on the left & click “Create New Protocol”. If you have had previous protocols submitted and approved, they will show up also on this page.
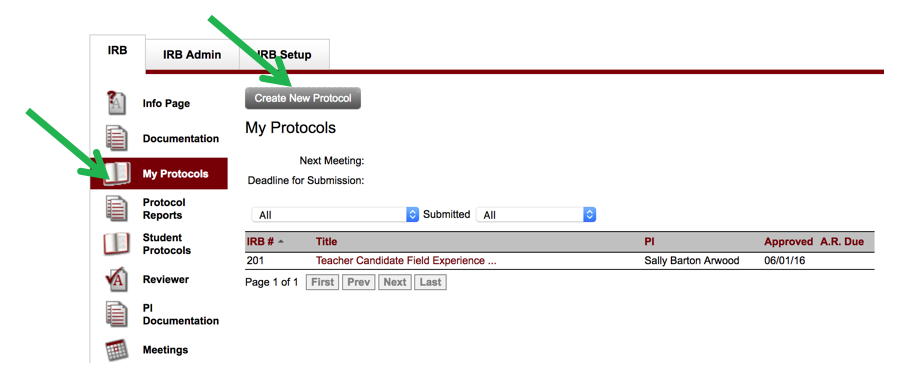
- NOTE: If you, as the PI, have not completed the CITI trainingand/oryour certificate is not in the Mentor system, you will see a message from the IRB chair regarding "PI Documentation, No Certificate." Please remember that the Mentor system will automatically block the submission of an IRB protocol if the PI does not have a certificate in Mentor. (Additionally, all co-PIs and faculty advisors of student research are required to complete the CITI training.) It is the responsibility of each researcher to upload their CITI training certificate under “PI documentation” in Axiom Mentor. It is also each researcher’s responsibility to make sure their certifications are still valid for the study duration and to complete refresher courses every 3 years.
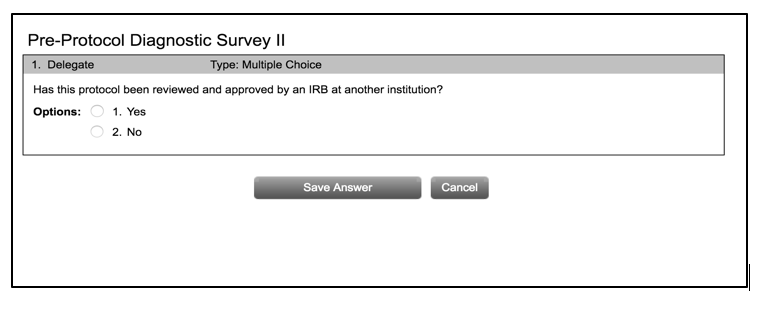
- Your application begins with the Pre-Protocol Diagnostic Survey, and you will be asked to answer a series of questions. The purpose of the survey is to help determine if your project needs IRB review, and if so, the appropriate level of review required. The IRB chair or designee will confirm. Please see the “Levels of Review” page on the Belmont IRB site for more information about the three different levels of review. Below is just the first question.
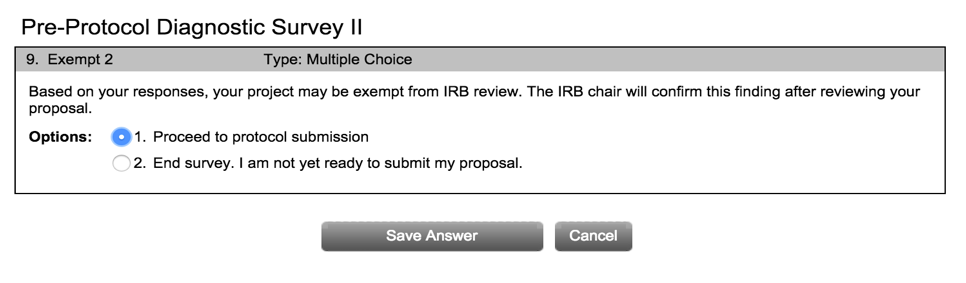
- Once the Pre-Protocol Diagnostic Survey has been completed, click “Proceed to protocol submission” to continue. No matter what level of review is determined (i.e., exempt, expedited or full), you will need to submit a completed application and appropriate consent form. These forms can be found under “Forms” on our IRB website. The IRB form and consent must be uploaded as a word document. Other documents may be PDF, but WORD is highly preferred.
- If you are conducting interviews or using surveys or questionnaires, be sure that the completed instrument(s) are uploaded as well. You may need to obtain approval from the author of the survey or questionnaire. Please be sure to get any necessary approvals before beginning your IRB submission.
- The final step, once all of your application is complete, is to click on “Submit Protocol for Review.”
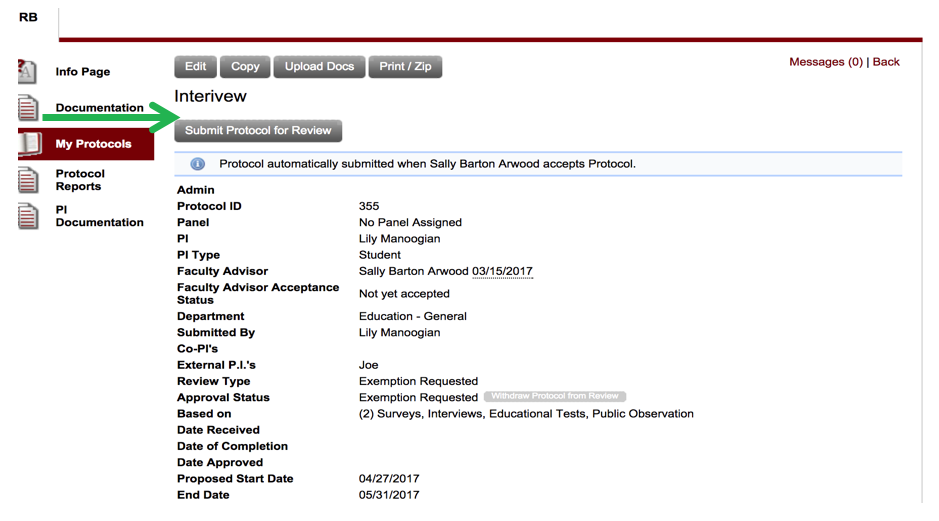
- It is very important that you understand that you may not begin work on your project until this entire review process is completed.
- Please note that the review of your protocol may be delayed due to the following errors:
- CITI certificates for all investigators involved with the study or faculty advisors of student research are not uploaded to the Mentor site.
- Only the pre-protocol survey is submitted and a completed application, which includes specific details of the study, is not included;
- Investigators do not carefully edit their protocols before submission ensuring all of the information is complete and the protocol and consent forms align (i.e., The protocol and consent forms are inconsistent in the procedures.). Also, please do a careful check for grammar and spelling on all documents.
- The application does not include the complete data collection tools (e.g., survey questions).
- The application does not include specific details regarding procedures for protecting the privacy of participants and maintaining the confidentiality of data.
- Consent form is written at too high of a reading level this is not within the recommended guidelines. Consents should be written at a 6th- 8th grade level.
Please note that this is not a complete list but some of the common situations that have slowed down reviews.
