As indicated in federal regulations for protection of human subjects (45 CFR part 46), investigators must obtain informed consent of the human subject or the subject’s legally authorized representation.
For protocols at expedited or full board review levels, documented informed consent will consist of a written consent form approved and stamp dated by the IRB and then signed and dated by the subject or the subject’s legally authorized representative. A copy shall be given to the person signing the form. The signed consent forms shall be kept in the investigator’s secured and protected files. (Participant signed consent forms should be maintained by the investigator for 3 years.)
The consent forms have been updated to align with the revised Common Rule. See the Forms section http://www.belmont.edu/irb/forms-instructions.html. The consent form for expedited and full board reviews is much different from the previous form. Federal Regulatons now require that informed consent begin with a concise and focused presentation of key information that will assist a prospective subject in understanding the reasons why one might or might not want to participate in the research. The consent form must also be organized and presented in a way that facilitates comprehension.
Informed consent is more than just getting participants to sign a form. It is a process that involves giving participants enough information so that they understand the research and its risks. The goal of the informed consent process is to provide sufficient information so that participants can make informed decisions about whether or not to originally participate in a study or to continue participation.
The process starts when investigators enroll participants in a study and is ongoing throughout the research project. Obtaining consent involves informing participants about their rights, the purpose of the project, the procedures that will occur during the study, and the potential risks and benefits of participation.
The informed consent document must be written in language easily understood by the participants (no higher than an 8th grade reading level). Additionally, participants must be given sufficient time to consider whether or not they will agree to participate. Within the process of informed consent, investigators must minimize the possibility of coercion or undue influence.
The Belmont University IRB provides investigators with an informed consent template. However, it is important to understand what must be included when gaining informed consent from your participants. Please see the end of this document for elements required for informed consent.
For participants not fluent in English, the consent process and document must be presented in a language (preferably native) understandable to them. If it is expected that participants who do not speak English will be enrolled in a study, translated documents should be made available.
Translation of Documents and Consent
Study related documents (e.g., the informed consent document, the HIPAA authorization,) may be translated by one of two methods. One method is that the document be translated by a professional translation service that will attest to the accuracy of the translation.
The second is the use of back-translation into English. In this scenario, the English version of the document is translated into the foreign language;
- the name and credentials of the individual who did the translation are provided to the IRB by the investigator;
- another individual who has not seen the English version of the document translates the foreign language document back into English;
- this individual provides his/her name, credentials and a statement that s/he has not seen the original English version to the IRB via the investigator;
- both English versions of the form and the foreign language version are submitted to the IRB for review.
The informed consent process must also be conducted in a language understandable to the participant and may therefore require the use of a translator or sign language interpreter. In most cases, the translator may be a family member or friend of the participant, an employee of the institution or may be hired by the PI. The IRB will determine whether a professional translator is required on a case-by-case basis.
The PI is responsible for covering the cost of the translation. The cost of the translation will not be incurred by the participants.
If one of the two recommended methods is not feasible, the IRB will accept certification from the PI that he/she or a member of the research staff translated the document and that the translation is accurate. This verification must accompany submission of the translated documents.
Signed informed consent is usually required; however, there are a few situations with the IRB can waive documentation of consent or waive consent
"Waiver of Documentation" of Informed Consent (see "What is implied Consent?" section")
A waiver of documentation of informed consent is a request whereby a signed consent document is not required. Examples include implied and verbal consent. Consent will still be obtained from participants; however, they will not be required to sign the consent form. There are only two circumstances when the IRB may waive the requirement to obtain a signed consent form:
- The only record linking the research participant and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality (participant must be asked if he/she want documentation); OR
- The research presents no more than minimal risk of harm to participants and involves no procedure for which written consent is normally required outside of the research context (for example, no risk surveys or interviews)
"Waiver" of Informed Consent? (How is it different from a "waiver of documentation" of informed consent?)
A waiver of informed consent could: (1) alter some or all of the required elements of informed consent or (2) completely waive the requirement to obtain informed consent. The IRB may approve a consent procedure which does not include or alters some or all of the required elements of informed consent provided all of the following are true:
- The research involves no more than minimal risk
- The waiver of informed consent will not adversely affect the rights and welfare of the subjects;
- It is not practicable to conduct the research without the waiver or alteration; or
- Whenever appropriate, participants will be provided with additional pertinent information after their participation.
Examples of types of studies in which some or all elements of consent have been waived include retrospective chart reviews, studies of existing pathology specimens, ethnographic research, studies that require deception or passive (opt-out) consent.
For some research projects, the IRB may approve a request to waive the documentation of informed consent. This means that the research team must provide a subject with the required consent information, but the study team is not required to obtain the subject's signature on the informed consent document. For example, if an investigator is conducting on online survey that has very little risk, by completing the survey, the participant has provided consent to participate in the research. The term "implied" consent is not referenced in the HHS regulations - see https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/informed-consent/index.htm.
Even in situations where the IRB may waive the documentation (signature) requirement (e.g., telephone interview, online survey), investigators are expected to present participants with the required key elements of informed consent and with a copy of the written consent document.
A waiver of documentation is permissible when:
- The signature on the informed consent document would be the only record linking the subject to the research and the principal risk of harm to the subject would be a breach of confidentiality. For example, for research on sensitive topics, such as domestic violence or illegal activities; OR
- The research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside the research context. For example, minimal risk research that invovles surveys/interviews conducted via telephone or online.
-
Under the 2018 Common Rule, there is an addtional requirement for the IRB approval of an informed consent documentation waiver request: Where the participants are members of a cultural group in which signing forms is not a normal/acceptable practice.
Consent forms are legal documents that can only be signed by adults, and assent forms give minors (i.e., children) the opportunity to convey their own independent decision to participate in research. Oral assent is appropriate for children ages 7 to 11 years. Written assent is required for children ages 12 to 15 and must be written in language-level appropriate for children. Assent alone is not sufficient to enroll a child in a study. The permission of a parent or guardian, using a parental permission form, must be obtained as well. The requirements for parental permission forms are very similar to those for consent forms. For children ages 16 and 17, a special consent form designed for both the child and parent to read and sign is required. The special consent form is also an available alternative to the written assent/parental permission option for children ages 12 to 15. A regular consent form should be used for individuals who are 18 years of age and older and have the decision-making capacity to consent. The informed consent form must be written at a language-level appropriate for the potential participants (i.e., about an 8th grade level). Please see the IRB webpage for templates for consent and child assent.
Elements of Informed Consent: See https://www.hhs.gov/ohrp/regulations-and-policy/guidance/checklists/index.html
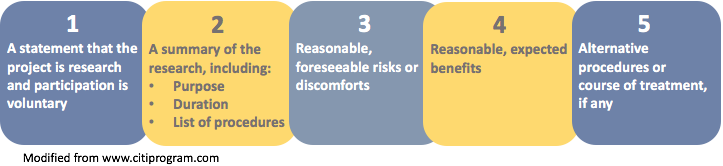
- A statement that the study involves research
- An explanation of the purposes of the research
- The expected duration of the subject's participation
- A description of the procedures to be followed
- Identification of any procedures which are experimental
- A description of any reasonably foreseeable risks or discomforts to the subject
- A description of any benefits to the subject or to others which may reasonably be expected from the research
- A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject
- A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained
- An explanation as to whether any compensation and an explanation as to whether any medical treatments are available, if injury occurs and, if so, what they consist of, or where further information may be obtained.
- Research, Rights or Injury:An explanation of whom to contact for answers to pertinent questions about the research and research subjects' rights, and whom to contact in the event of a research-related injury to the subject
- A statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits, to which the subject is otherwise entitled
Additional Elements as Appropriate
- A statement that the particular treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant), which are currently unforeseeable
- Anticipated circumstances under which the subject's participation may be terminated by the investigator without regard to the subject's consent
- Any additional costs to the subject that may result from participation in the research
- The consequences of a subject's decision to withdraw from the research and procedures for orderly termination of participation by the subject
- A statement that significant new findings developed during the course of the research, which may relate to the subject's willingness to continue participation, will be provided to the subject
- The approximate number of subjects involved in the study